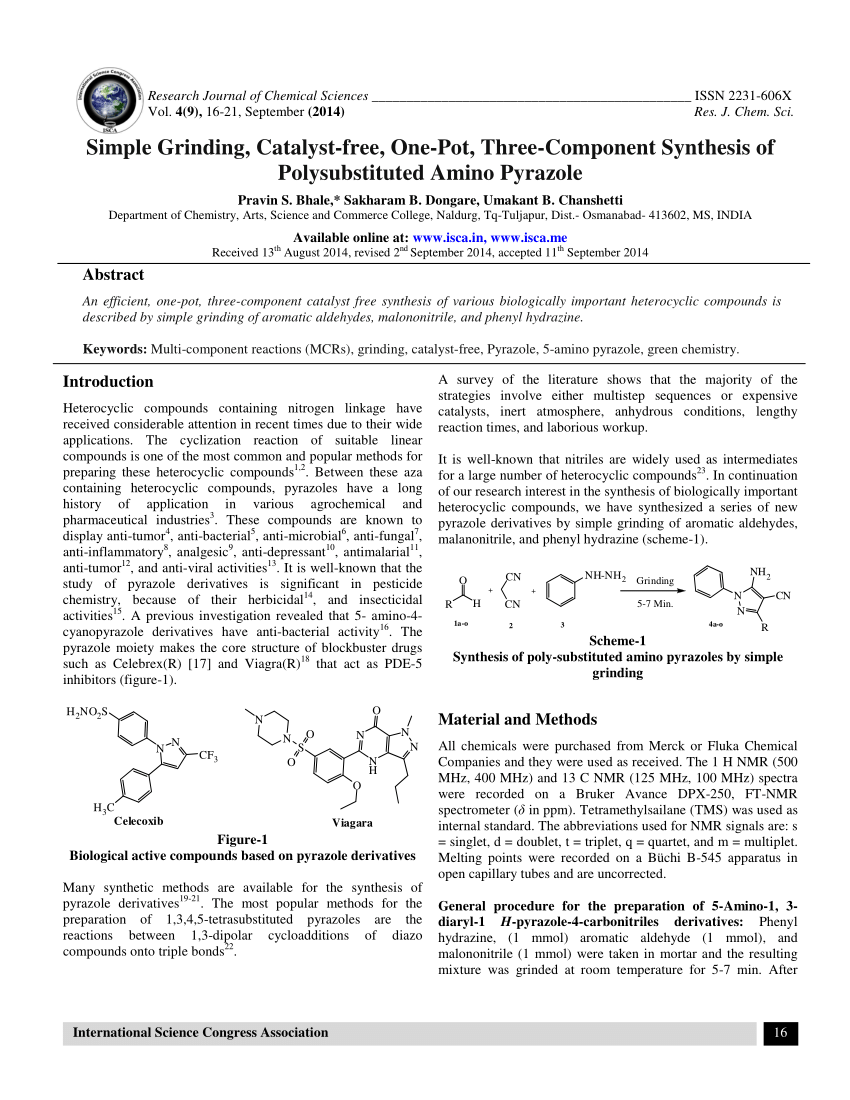
-
Chemistry Of Pyrazole Pdf카테고리 없음 2020. 3. 5. 07:03
ABOUT AUTHORS:Vishwanadham Yerragunta. 1, Duggi.Suman 1, Kumara swamy 1, V.Anusha 1, Pratima Patil 1, M.
Naresh 2Department of Pharmaceutical Chemistry,1Malla Reddy College of Pharmacy, Maisammaguda, Secunderabad-500014, A.P.2Bharath Institute of Technology Pharmacy-Ibrahimpatnam, AP.ABSTRACT:The aim of this review is to provide an overview of diverse pharmacological activities of pyrazole moiety. Pyrazole are well known and important nitrogen containing 5-membered heterocyclic compounds and various methods have been worked out for their synthesis. Pyrazole chemically known as 1, 2-diazole has become a popular topic due to its manifold uses. Numerous pyrazole derivatives have been found to possess a broad spectrum of biological activities, which stimulated the research activity in this field.

Pyrazoles and its derivatives represent one of the most active classes of compounds, which possess wide range of biological activities like anti-bacterial, anti-convulsant, analgesic, anti-microbial, anti-inflammatory, ant diabetic, sedative anti-rheumatic, anticancer, and anti-tubercular activities. The purpose of this review was to collate literature work reported by researchers on pyrazole for their various pharmacological activities and also reported recent efforts made on this moiety. REFERENCE ID: PHARMATUTOR-ART-2073PharmaTutor (ISSN: 2347 - 7881)Volume 2, Issue 1Received On:; Accepted On:; Published On: How to cite this article: V Yerragunta, D Suman, K swamy, V Anusha, P Patil, M Naresh, Pyrazole and Its Biological Activity, PharmaTutor, 2014, 2(1), 40-48INTRODUCTION:The chemistry of pyrazoles has been extensively investigated in the past. Pyrazoles are five member ring heterocyclic compounds, have some structural features with two nitrogen atoms in adjacent position and are also called as azoles 1.The chemical reactivity of the pyrazole molecule can be explained by the effect of individual atoms.
Chemistry Of Pyrazole Pdf Online
The N-atom at position 2 with two electrons is basic and therefore reacts with electrophiles. The N-atom at position 1 is unreactive, but loses its proton in the presence of base. The combined two N-atoms reduce the charge density at C3 and C5, making C4 available for electrophilic attack. Deprotonation at C3can occur in the presence of strong base, leading to ring opening. Protonation of pyrazoles leads to pyrazolium cations that are less likely to undergo electrophilic attack at C4, but attack at C3 is facilitated. The pyrazole anion is much less reactive toward nucleophiles, but the reactivity to electrophiles is increased 2Pyrazoles are aromatic molecules due to their planar conjugated ring structures with six delocalized π-electrons. Therefore, many important properties of these molecules were analyzed by comparing with the properties of benzene derivatives 3.
Like other nitrogen involving heterocycles, different tautomeric structures can be written for pyrazoles. Unsubstituted pyrazole can be represented in three tautomeric forms 4. Tautomeric forms of unsubstituted pyrazole.Now a day’s vast number of compounds with pyrazole nucleus have been reported to show a broad spectrum of biological activity including.
Antimicrobial 5, antiviral 6, anti-tumor 7,8, anti-histaminic 9, anti-depressant 10, insecticides 11 and fungicides 11. Due to its wide range of biological activity, pyrazoles ring constitutes a relevant synthetic route in pharmaceutical industry. In fact, such a heterocyclic moiety represents the core structure for number of drugsLITERATURE: Eman M. Flefel et.al have reported the new substituted pyrazole, thiazole, and 1, 2, 4-triazole derivatives were synthesized. The sugar hydrazones, their acetylated derivatives as well as their derived acyclic C-nucleoside analogs, and the thioglycosides of the 1, 2, 4-traizole derivatives were also prepared. The antitumor activity of some of the synthesized compounds were studied, and a number of the tested compounds showed significant activities 12. Mohamedsalahk.youssef et.al., have synthesized Ethyl 7-amino-3-(3-methyl-5-oxo-1-phenyl-2-pyrazolin-4-yl)-5-aryl-5 H-thiazolo3,2- apyrimidine-6-carboxylate was synthesized by the reaction of 4-(2-aminothiazol-4-yl)-3-methyl-5-oxo-1-phenyl-2-pyrazoline with arylidene ethyl cyanoacetate and it transformed to related fused heterocyclic systems via reaction with various reagents 13.
Rao Jyothi et al., have synthesized a novel series of 1, 3, 5-trisubstituted pyrazoles by the cyclo condensation reaction of chalcones and substituted hydrazides by irradiation under microwave energy and also by conventional method. Compound 3g showed good activity against E. Coli and P.aerugiosa. Compound 3j showed good activity against the fungus A. Fumigatus 14. Mishra et al., have reported the synthesis of a series of 1- (2, 4-dinitrophenyl)-3-(3-nitrophenyl)-5-(4-substituted phenyl)-2-pyrazolin-4-ones by the oxidation of 1-(2, 4-dinitrophenyl)-3-(3-nitrophenyl)-5-(4-substituted phenyl) - 4-bromo-2-pyrazolines with dimethyl-sulfoxide and assayed for in vitro antimicrobial activity.
Most of the synthesized compounds did not exhibit significant inhibitory activity against the tested strains 15. SatheeshaRai N and BalakrishnaKalluraya et.al., have reported novel series of nitrofuran containing 1,3,4,5 tetra substituted pyrazole derivatives.
Compound 3b showed highest anti-bacterial and antifungal activity than all other compounds 16. Sahu SK et al., have synthesized a series of novel 4-(5-substituted aryl-4, 5-dihydropyrazole-3-yl-amino) phenols by treating substituted aryl-N-chalconyl amino phenols with hydrazine hydrate. It was observed increase in analgesic, anti-inflammatory and anti -microbial activities are attributed to the presence of 4-NO2, 2-OH and 4-Cl in phenyl ring at 5-position of pyrazoline ring of synthesized compounds 17.NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.SUBMIT YOUR ARTICLE/PROJECT ATFIND OUT AT OUR DATABASE.
Chemistry Of Pyrazole Pdf List
Except where otherwise noted, data are given for materials in their (at 25 °C 77 °F, 100 kPa).Y ( Y N?)Pyrazole is an organic compound with the C 3H 3N 2H. It is a characterized by a 5-membered ring of three atoms and two adjacent nitrogen atoms. Pyrazole is a weak base, with p K b 11.5 (p K a of the conjugated acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C 3N 2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are (Celebrex) and the anabolic steroid. Novel pyrazole ligands History The term pyrazole was given to this class of compounds by German Chemist in 1883.

In a classical method developed by German chemist in 1898, pyrazole was synthesized from. Conversion to scorpionates Pyrazoles react with to form a class of ligands known as. Pyrazole itself reacts with at high temperatures (200 °C) to form a known as:3,5-Diphenyl-1 H-pyrazole 3,5-Diphenyl-1 H-pyrazole is produced when is reacted with in the presence of elemental or, or by using a in which case an is produced as a by-product. Occurrence and uses. A pyrazole derivative used as an analgesicIn 1959, the first natural pyrazole, was isolated from seeds of.In medicine, derivatives of pyrazole are widely usedThe pyrazole ring is found within a variety of pesticides as fungicides, insecticides and herbicides, including, and. Pyrazole moieties are listed among the highly used ring systems for small molecule drugs by the USFDASee also., an analog of pyrazole with two non-adjacent nitrogen atoms., another analog, the nitrogen atom in position 1 replaced by oxygen.References.